New publication in 2022: Heat and Mass Transfer (IF: 5.584)
Abstract
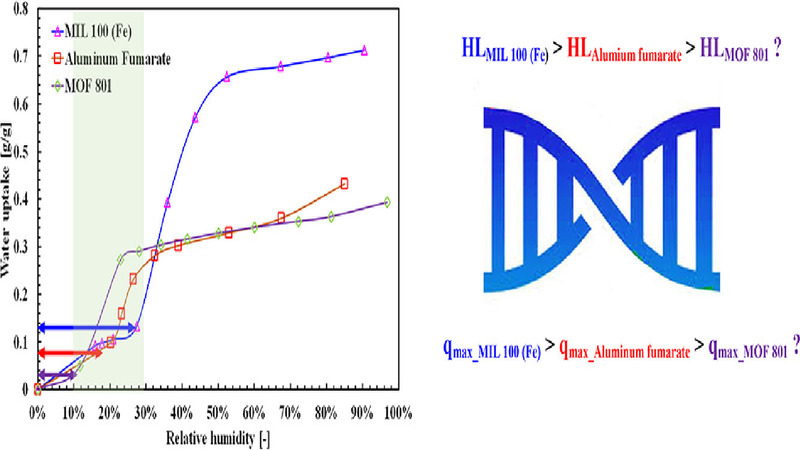
The usage of metal organic frameworks (MOFs) as adsorbent materials for adsorption-based heat transformation applications is gaining popularity among the research society due to their S-shaped water adsorption isotherms. Apart from the S-shaped isotherms, there are other two key features that need to be kept in mind when working with MOF/water pair, namely the equilibrium uptake and the hydrophobic length of the water adsorption isotherms. Therefore, in this study, three different metal organic frameworks, namely MOF–801, aluminum fumarate, and MIL–100(Fe), are synthesized, and water adsorption onto these adsorbents is measured thermogravimetrically. Utilizing the four-step temperature swing adsorption process, the entropy generation in each step is calculated for each pair. Surface properties of the MOFs are measured using the inverse gas chromatography method. We explicitly demonstrated the relation between surface energy components of the adsorbents and their respective water adsorption isotherms. It has been found that the equilibrium uptake is proportional to the surfaces’ work of adhesions, while the hydrophobic length shows a relation with the surface acid-base properties. This information is crucial for designing an optimum MOF adsorbent for an efficient adsorption-based heat transformation application.