New publication in 2022: Thermal Science and Engineering Progress (IF: 4.56 )
Abstract
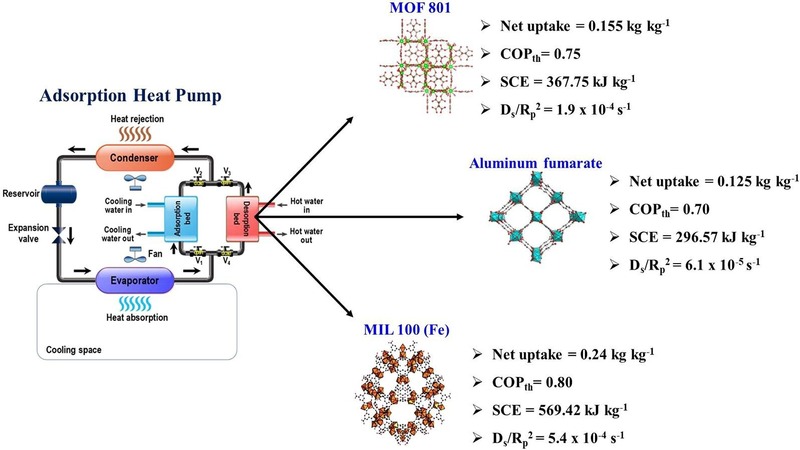
MOFs (metal organic frameworks), a new type of porous coordination polymers (PCPs), has emerged as a novel class of adsorbent material that has piqued the interest of academics working on a variety of sorption-related applications all over the world. Adsorption heat pump research is no exception, and it has accelerated since the MOF adsorbents were introduced. In this study, water adsorption isotherms onto three distinct synthesized MOFs, MOF 801, aluminum fumarate, and MIL 100 (Fe), were experimentally obtained at three different temperatures (30, 50, and 70 °C) with varying evaporating pressures using the thermogravimetric technique. The S-shaped adsorption isotherms were correlated with the well-known Sun-Chakraborty model. P-T-W diagrams were generated using the adsorption isotherms data to determine the net adsorption uptake within a certain adsorption heat pump working condition. Water sorption kinetics were also analyzed using the time-adapted linear driving force model. Adsorption enthalpy, specific cooling effect, and coefficient of performance of the studied pairs were calculated and compared with the results of the PS-I/water pair. Results suggested that, for a water-based adsorption cooling system, the studied MOF adsorbents significantly outperform the traditional polymer-based adsorbent PS-I. Among the MOF/water pairs, MIL 100 (Fe)/water pair showed the fastest sorption kinetics with a diffusion time constant of 5.4 × 10−4 s−1. This pair also exhibited the highest specific cooling effect of 569.42 kJ kg−1 and a coefficient of performance of 0.80, which was rather impressive. These results will be valuable in determining suitable adsorbent-adsorbate pairs for next-generation adsorption cooling systems.