New publication in 2022: Thermal Science and Engineering Progress (IF: 4.56 )
Abstract
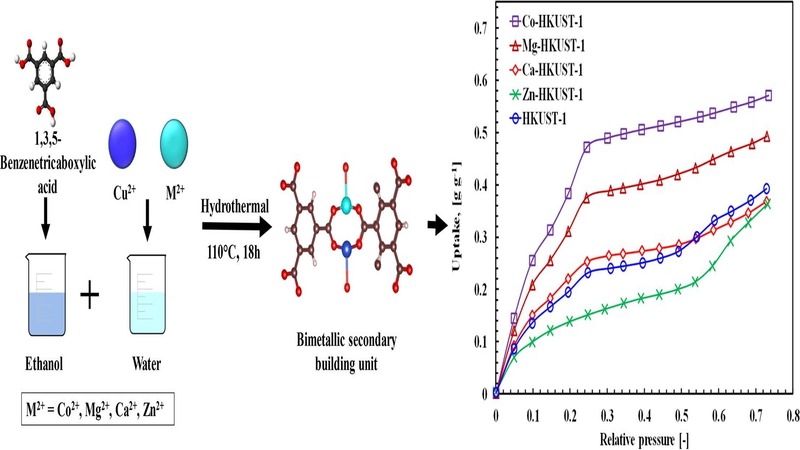
Metal-organic frameworks (MOFs) are inorganic–organic hybrid porous crystalline materials featured by exceptionally high surface area, tunable porosity, and controllable chemical structures, making them efficient candidates for gas adsorption. HKUST-1 is a promising MOF constructed from the coordination bonding of copper paddle-wheel metal nodes and 1,3,5-benzenetricarboxylates. In this study, HKUST-1 was synthesized according to a hydrothermal synthesis procedure, and two alkaline metal ions (Ca2+ and Mg2+) and two transitional metal ions (Co2+ and Zn2+) were individually doped in situ in the framework of HKUST-1. The synthesized pristine HKUST-1 and the metal-doped MM-HKUST-1 were then characterized experimentally. MM-HKUST-1 samples were isostructural with the pristine MOF, and larger surface area and increased pore volume were observed due to metal doping. The effect of the presence of different metals on the water sorption capacity was investigated by volumetric water sorption analysis. The incorporation of metal ions Co2+ and Mg2+ enhanced the affinity towards water vapor, whereas the equilibrium uptake capacity of the other two metal-doped samples was comparable with the parent one. The increased water uptake capacity eventually increased the SCE of the Co-HKUST-1 sample for providing cooling of 15 °C with heat source temperatures of 50 °C and 60 °C.